Objective
The purpose is to assess how treatment with recombinant human growth hormone (rhGH; Omnitrope®) impacts the possibility of developing diabetes mellitus in adults diagnosed with growth hormone deficiency (GHD) by analyzing data from the PATRO Adults post-marketing surveillance study.
Methods
All adult patients over 30 years who have received more than or equal to one dose of Omnitrope® in a post-marketing surveillance study currently ongoing in different European hospitals and endocrinology clinics have been categorized under the safety population. The study is called PATRO Adults. Patient profiles who had adverse events of diabetes mellitus during the study were generated. Diabetes mellitus was confirmed if glycated hemoglobin ≥6.5% or 2-h plasma glucose ≥11.1 mmol/L or the fasting plasma glucose was ≥7.0 mmol/L when administered with an oral glucose test.
Results
As of July 2018, there were 1,293 participants in the study, with 983 (76.0%) continuing to be active. Almost half of these adult patients (n = 687, 49.3%) had not previously undergone growth hormone (GH) treatment. The majority (n = 1,128, 87.2%) were diagnosed with multiple pituitary hormone deficiency (MPHD). In 21 adult patients or 22 events, inadequate control or worsening of diabetes mellitus was reported. There were new diagnoses in 15 adult patients between ages 29 and 84 years (incidence rate 3.61 per 1000 patient-years). 6 of the cases were adult patients aged between 45 and 75 years with existing diabetes mellitus conditions at baseline.
The new occurrence of diabetes mellitus was primarily seen in adult patients with adult-onset MPHD (n = 13). The rest of the cases of a new occurrence of diabetes mellitus were in adult patients with childhood-onset MPHD, and they had received the GH replacement therapy earlier (n=1). Another patient developed isolated GHD in adulthood and had not undergone GH replacement therapy in the past (n = 1).
Every case of worsening or inadequate control of diabetes mellitus was observed in adult patients with adult-onset MPHD.
Conclusions
Based on the PATRO Adults study, there are indications that adult patients with GHD occurrences can tolerate Omnitrope® treatment in real-world clinical settings. Thus far, there have been no indications of any increased risk for diabetes mellitus, though ongoing monitoring (both during and post-RhGH therapy) is a must to verify these findings. Even with encouraging results, it is to be noted that Omnitrope® treatment is not a concoction or a promise of perpetual youth or standardized results.
Background
Growth hormone deficiency (GHD) in adults is an observed medical condition. The estimated prevalence is between 2 and 3 cases per 10,000. It is commonly marked by negative impacts on body composition, leading to increased abdominal fat, decreased muscle mass, and lower strength in skeletal muscles. Moreover, GHD in adults is linked to weakened lipid and carbohydrate metabolism, contributing to a higher likelihood of cardiovascular complications that are primarily attributed to the rise in visceral fat. Additionally, the quality of life (QoL) for adults suffering from GHD often declines, as symptoms like depression, fatigue, anxiety, social withdrawal, and a decrease in physical activity levels are witnessed in such adults.
Growth hormone (GH) has a vital role in glucose regulation. In adults suffering from GHD, abnormalities in glucose metabolism, like insulin resistance and increased fasting insulin levels, have been reported. Abdominal obesity is common in GHD adult patients and is believed to be a significant contributor to the decreased insulin sensitivity seen in many individuals. Additionally, adults with GHD issues have higher risks of developing diabetes mellitus than those without GHD. The risks are higher in those with a family history of obesity or diabetes mellitus.
GH replacement therapy has been designed to address dysfunctional metabolic, functional, and psychological issues that are linked to GHD. Recombinant human GH (rhGH) can improve body composition effectively (by reducing fat mass and increasing lean body mass). It also improves exercise capacity, blood lipid profiles, and the quality of life in adults with GHD. Initially, three intramuscular injection doses of higher concentration were given weekly during the rhGH therapy. The concentrations were fixed as per the body weight of the patient. The three doses usually decreased insulin sensitivity temporarily. After the first year of treatment, the insulin levels would normalize.
Since the 1990s, however, the injections are given daily. These subcutaneous injections align with insulin-like growth factor-I (IGF-I) levels. These are adjusted as per age and gender. Consequently, the doses are usually lower, and the side effects are reduced. Studies show that insulin sensitivity and resistance usually stay stable with low-dose, long-term rhGH treatment.
GH replacement therapy studies for adults indicate that HbA1c levels stay consistent, though one study noted a slight reduction in the patient after 15 years of GH therapy. That's why it is advisable to regularly monitor HbA1c levels in adult GHD patients, especially if one is at a higher risk for diabetes mellitus or if the person has already been diagnosed with the condition. Hypoglycemic medications may be adjusted accordingly based on these monitoring outcomes.
Omnitrope® from Sandoz is a biosimilar version of recombinant human growth hormone (rhGH) and has been approved by the European Medicines Agency in 2006 for its quality, safety, and efficacy. All the parameters matched those of the reference medication, Genotropin® (Pfizer). It is used to treat significant growth hormone deficiency (GHD) in adults, whether it began in childhood or during adult life. The adult patients Treated with Omnitrope® (PATRO) Adults study is an ongoing post-marketing surveillance project on European hospitals and specialized endocrinology clinics.
Since Omnitrope®'s status is an approved biosimilar rhGH in Europe, this study plays a crucial role in assessing the long-term safety profile in adults with GHD vis-a-vis that of its reference medication. The PATRO Adults study aims to understand the long-term safety of Omnitrope® when used in a standard clinical setting. The secondary objective is to track the effectiveness of the treatment through various measures, like insulin-like growth factor-I (IGF-I) levels, lipid profiles, and body composition changes.

- Human Growth Hormone Injections In Cheyenne [Last Updated On: May 25th, 2025] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Madison [Last Updated On: May 31st, 2025] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Vancouver [Last Updated On: February 24th, 2025] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Tacoma [Last Updated On: February 25th, 2025] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Spokane [Last Updated On: February 26th, 2025] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Bellevue [Last Updated On: February 26th, 2025] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Richmond [Last Updated On: May 31st, 2025] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Portsmouth [Last Updated On: June 1st, 2025] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Norfolk [Last Updated On: June 1st, 2025] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Hampton [Last Updated On: March 27th, 2025] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Chesapeake [Last Updated On: March 27th, 2025] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Alexandria [Last Updated On: March 28th, 2025] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Montpelier [Last Updated On: June 2nd, 2025] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Provo [Last Updated On: June 2nd, 2025] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Waco [Last Updated On: June 7th, 2025] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Richardson [Last Updated On: June 5th, 2025] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Plano [Last Updated On: June 6th, 2025] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Pasadena [Last Updated On: June 4th, 2025] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Midland [Last Updated On: June 7th, 2025] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Mesquite [Last Updated On: June 4th, 2025] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In McKinney [Last Updated On: June 3rd, 2025] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In McAllen [Last Updated On: June 8th, 2025] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Lubbock [Last Updated On: June 6th, 2025] [Originally Added On: October 19th, 2018]
- Human Growth Hormone Injections In Lewisville [Last Updated On: June 5th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Laredo [Last Updated On: June 3rd, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Killeen [Last Updated On: February 18th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Irving [Last Updated On: February 19th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Garland [Last Updated On: February 20th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Denton [Last Updated On: February 21st, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Carrollton [Last Updated On: June 8th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Brownsville [Last Updated On: February 21st, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Beaumont [Last Updated On: February 18th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Austin [Last Updated On: February 22nd, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Arlington [Last Updated On: February 20th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Amarillo [Last Updated On: February 19th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Abilene [Last Updated On: February 22nd, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Nashville [Last Updated On: February 27th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Murfreesboro [Last Updated On: February 27th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Memphis [Last Updated On: February 28th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Knoxville [Last Updated On: February 28th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Clarksville [Last Updated On: March 1st, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Chattanooga [Last Updated On: March 1st, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Erie [Last Updated On: March 29th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Allentown [Last Updated On: March 28th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Salem [Last Updated On: March 2nd, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Portland [Last Updated On: March 2nd, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Gresham [Last Updated On: March 3rd, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Eugene [Last Updated On: March 3rd, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Tulsa [Last Updated On: March 4th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Norman [Last Updated On: March 4th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Toledo [Last Updated On: May 27th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Dayton [Last Updated On: May 29th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Columbus [Last Updated On: May 29th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Akron [Last Updated On: May 30th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Paterson [Last Updated On: February 23rd, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Reno [Last Updated On: February 24th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Henderson [Last Updated On: February 25th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Omaha [Last Updated On: May 26th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Lincoln [Last Updated On: May 26th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Billings [Last Updated On: May 27th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Springfield [Last Updated On: May 28th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Independence [Last Updated On: August 30th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Columbia [Last Updated On: May 28th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Jackson [Last Updated On: May 30th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Rochester [Last Updated On: February 23rd, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Warren [Last Updated On: March 5th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Lansing [Last Updated On: March 5th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Flint [Last Updated On: March 6th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Worcester [Last Updated On: March 29th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Springfield [Last Updated On: March 30th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Lowell [Last Updated On: March 30th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Cambridge [Last Updated On: March 31st, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Augusta [Last Updated On: March 6th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Shreveport [Last Updated On: March 7th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Lafayette [Last Updated On: March 7th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Louisville [Last Updated On: March 8th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Lexington [Last Updated On: March 8th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Wichita [Last Updated On: March 9th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Topeka [Last Updated On: March 9th, 2025] [Originally Added On: October 20th, 2018]
- Human Growth Hormone Injections In Olathe [Last Updated On: March 10th, 2025] [Originally Added On: October 20th, 2018]

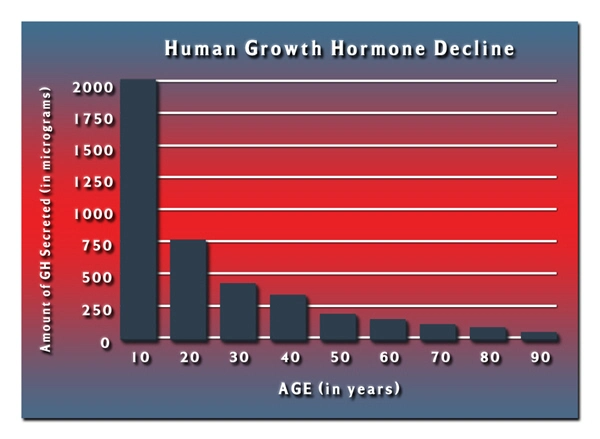
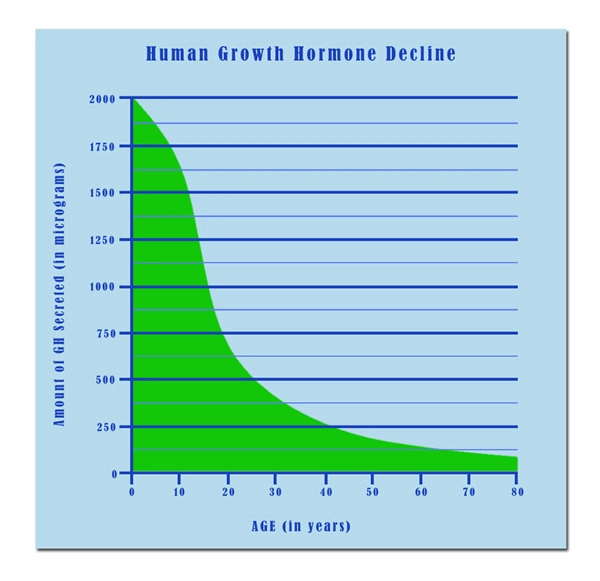
List of USA state clinics - click a flag below for blood testing clinics.
Word Count: 957