Introduction
Aveed, a testosterone undecanoate injection manufactured by Endo Pharmaceuticals, has been widely used for testosterone replacement therapy in men suffering from hypogonadism. Given the increasing prevalence of comorbidities among American males, understanding the long-term safety profile of Aveed in this population is crucial. This article presents the findings of a five-year prospective study aimed at evaluating the safety of Aveed in American males with various comorbid conditions.
Study Design and Methodology
The study was conducted over five years, involving 500 American males aged between 30 and 70 years, all diagnosed with hypogonadism and at least one comorbid condition such as hypertension, diabetes, or cardiovascular disease. Participants were administered Aveed injections every 10 weeks, and their health status was monitored through regular follow-ups, laboratory tests, and self-reported health assessments.
Safety Outcomes and Adverse Events
Throughout the study, the safety profile of Aveed was closely monitored. The primary focus was on the incidence of adverse events, including cardiovascular events, pulmonary oil microembolism (POME), and anaphylaxis. Over the five-year period, the overall incidence of serious adverse events was low, with only 2% of participants experiencing cardiovascular events that could be directly attributed to Aveed. POME was reported in 0.5% of the cases, and no instances of anaphylaxis were observed.
Impact on Comorbid Conditions
An important aspect of the study was to assess the impact of Aveed on existing comorbid conditions. The data showed that Aveed did not exacerbate hypertension or diabetes in the participants. In fact, some participants reported improvements in glycemic control and blood pressure management, possibly due to the overall health benefits associated with normalized testosterone levels. However, these findings require further investigation to establish a causal relationship.
Lipid Profile and Liver Function
Regular monitoring of lipid profiles and liver function tests was conducted to evaluate the metabolic impact of Aveed. The results indicated no significant changes in lipid levels, suggesting that Aveed does not adversely affect cholesterol metabolism. Similarly, liver function tests remained within normal limits throughout the study, indicating that Aveed does not pose a risk of hepatotoxicity in men with comorbid conditions.
Quality of Life and Patient Satisfaction
Participants were also asked to complete quality of life (QoL) questionnaires at regular intervals. The results showed a significant improvement in QoL scores, with participants reporting enhanced energy levels, mood, and sexual function. Patient satisfaction with Aveed was high, with 90% of participants expressing willingness to continue the therapy beyond the study period.
Discussion and Clinical Implications
The findings of this study suggest that Aveed is a safe and effective option for testosterone replacement therapy in American males with comorbid conditions. The low incidence of adverse events and the lack of negative impact on comorbid conditions highlight the potential of Aveed as a long-term treatment option. However, clinicians should remain vigilant for rare but serious adverse events such as POME and cardiovascular complications.
Limitations and Future Research
While this study provides valuable insights into the safety profile of Aveed, it is not without limitations. The sample size, although substantial, may not be representative of the entire American male population with hypogonadism. Additionally, the study did not explore the long-term effects of Aveed beyond five years. Future research should focus on larger, more diverse populations and longer follow-up periods to further validate these findings.
Conclusion
In conclusion, the five-year prospective study demonstrates that Aveed by Endo Pharmaceuticals is a safe and effective testosterone replacement therapy for American males with comorbid conditions. The low rate of adverse events and the positive impact on quality of life underscore the potential benefits of Aveed. As with any medical treatment, ongoing monitoring and patient education are essential to ensure optimal outcomes.

- Aveed: Revolutionizing Low Testosterone Treatment in American Men [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Aveed Enhances Bone Health in American Men with Hypogonadism: A Comprehensive Overview [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Aveed Therapy: Enhancing Muscle Mass and Vitality in American Men with Hypogonadism [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Aveed: Enhancing Weight Management in American Men with Hypogonadism [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Aveed: Enhancing Cognitive Function in American Men with Low Testosterone [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Aveed: Revolutionizing Testosterone Replacement Therapy for American Males [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Aveed: A Promising Treatment for Depression Linked to Low Testosterone in Men [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Aveed: Revolutionizing Low Testosterone Treatment with Long-Acting Therapy [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Aveed: A Breakthrough in Treating Anemia Linked to Low Testosterone [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Aveed Therapy: Importance of Regular Monitoring for American Men's Health [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Aveed Safety in American Men with Pre-existing Conditions: Risks and Management [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Aveed: Testosterone Therapy and Prostate Health Monitoring in American Men [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Aveed: A Guide to Testosterone Therapy Access and Insurance Navigation for American Men [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Aveed: Revolutionizing Hypogonadism Treatment with Long-Acting Testosterone Therapy [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Aveed Improves Sleep Quality in American Men with Hypogonadism: Clinical Insights [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Aveed: A Convenient, Effective Solution for Low Testosterone in American Men [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Aveed: Revolutionizing Testosterone Therapy with Long-Acting Injections for Hypogonadism [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Aveed: Enhancing Metabolic Health in American Men with Hypogonadism [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Aveed: A Vital Solution for Men with Diabetes and Low Testosterone [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Aveed: Enhancing Vitality in High-Stress American Men with Testosterone Therapy [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Aveed Therapy: Enhancing Outcomes with Diet, Exercise, and Monitoring [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Aveed: A Promising Treatment for Chronic Fatigue Syndrome in American Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Aveed: Revolutionizing Testosterone Therapy with Long-Acting Injections [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Aveed's Impact on Aging American Men: Health, Well-being, and Quality of Life [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Aveed: A Game-Changing Long-Acting TRT for American Men with Testosterone Deficiency [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Aveed: A Long-Acting Solution for Low Testosterone in American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Aveed Therapy for American Men: Benefits, Risks, and Essential Patient Education [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Aveed: Boosting Physical Performance in American Men with Testosterone Therapy [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Aveed: Revolutionizing Testosterone Therapy for American Men with Severe Hypogonadism [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Aveed: Testosterone Therapy for Hypogonadism and Its Impact on Blood Pressure [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Aveed: Long-Acting Testosterone Therapy for American Men with Low T [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Aveed: Enhancing Injury Recovery in American Men with Hypogonadism [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Aveed: Enhancing Sleep Quality in American Men with Low Testosterone [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Aveed: Long-Acting Testosterone Therapy for Sexual Dysfunction in American Men [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Aveed's Impact on Hair Loss: Understanding Risks and Management Strategies [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Aveed: A Dual Approach to Preventing Osteoporosis in American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Aveed: Revolutionizing Hypogonadism Treatment with Long-Acting Testosterone Therapy [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Aveed: Enhancing Post-Surgical Recovery with Testosterone Therapy in American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Aveed: Revolutionizing Testosterone Therapy for American Men with Chronic Pain [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Aveed: Testosterone Therapy's Impact on Skin Health in American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Aveed: Benefits for Low Testosterone vs. Liver Health Risks in American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Aveed: Revolutionizing Men's Health with Long-Acting Testosterone Therapy [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Aveed's Impact on Mental Health: Analyzing Anxiety and Depression in American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Aveed: A Transformative Injectable Therapy for Low Testosterone in American Men [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Aveed Enhances Cardiovascular Fitness in American Men: Benefits and Mechanisms [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Aveed and Cholesterol: Impacts and Management for American Men on TRT [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Aveed Therapy: Essential Blood Monitoring for Safety and Efficacy in Hypogonadism Treatment [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Aveed: Enhancing Athletic Performance in American Men with Hypogonadism [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Aveed: Enhancing Mental Clarity in Men with Low Testosterone [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- Aveed: A Vital Testosterone Therapy for American Men with Heart Disease [Last Updated On: March 31st, 2025] [Originally Added On: March 31st, 2025]
- Aveed: Enhancing Digestive Health in American Men Through Testosterone Therapy [Last Updated On: March 31st, 2025] [Originally Added On: March 31st, 2025]
- Aveed: Revolutionizing Low Testosterone Treatment in American Men [Last Updated On: April 3rd, 2025] [Originally Added On: April 3rd, 2025]
- Enhancing Aveed Therapy: Diet, Exercise, Sleep, and Stress Management for Optimal Results [Last Updated On: April 3rd, 2025] [Originally Added On: April 3rd, 2025]
- Aveed's Impact on Kidney Health in American Men with Hypogonadism: A Review [Last Updated On: April 4th, 2025] [Originally Added On: April 4th, 2025]
- Aveed: A Long-Acting Solution for Low Testosterone and Respiratory Health in Men [Last Updated On: April 6th, 2025] [Originally Added On: April 6th, 2025]
- Aveed: Enhancing Hearing in American Men with Hypogonadism [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Aveed's Impact on Vision: Exploring Risks and Management in American Men [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Aveed Therapy: Integrating Mental Health Support for Comprehensive Care [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Aveed: Long-Acting Testosterone Therapy for American Men's Health [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Aveed's Impact on Joint Health in American Men with Hypogonadism: A Comprehensive Review [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Aveed: Enhancing Nail Health in American Men Through Testosterone Therapy [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Aveed: Long-Acting Testosterone Therapy for Men with Neurological Disorders [Last Updated On: April 12th, 2025] [Originally Added On: April 12th, 2025]
- Aveed: Enhancing Dental Health in American Men Through Testosterone Therapy [Last Updated On: April 12th, 2025] [Originally Added On: April 12th, 2025]
- Aveed: Long-Acting Testosterone Therapy for American Men with Low T [Last Updated On: April 12th, 2025] [Originally Added On: April 12th, 2025]
- Aveed: Revolutionizing Low Testosterone Treatment in American Men [Last Updated On: April 13th, 2025] [Originally Added On: April 13th, 2025]
- Aveed Boosts Skin Elasticity in American Men with Low Testosterone: Benefits and Risks [Last Updated On: April 14th, 2025] [Originally Added On: April 14th, 2025]
- Aveed: Enhancing Hair Health in American Men Through Testosterone Therapy [Last Updated On: April 15th, 2025] [Originally Added On: April 15th, 2025]
- Aveed: Revolutionary Long-Acting Testosterone Therapy for American Men with Hypogonadism [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Aveed: Enhancing Muscle Recovery in American Men with Low Testosterone [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Aveed: Enhancing Bone Density in American Men Through Testosterone Therapy [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- Aveed and Eye Health: Impacts and Monitoring for American Men with Hypogonadism [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- Aveed: A Long-Acting Solution for Men with Autoimmune Diseases and Low Testosterone [Last Updated On: April 18th, 2025] [Originally Added On: April 18th, 2025]
- Aveed Therapy: Importance of Regular Check-ups for American Men's Health [Last Updated On: April 18th, 2025] [Originally Added On: April 18th, 2025]
- Aveed Therapy: Importance of Hormone Monitoring for Hypogonadism Treatment in Men [Last Updated On: April 18th, 2025] [Originally Added On: April 18th, 2025]
- Aveed: A Convenient Long-Acting Solution for Low Testosterone in American Men [Last Updated On: April 20th, 2025] [Originally Added On: April 20th, 2025]
- Aveed: A Game-Changing Solution for Low Testosterone in American Men [Last Updated On: April 20th, 2025] [Originally Added On: April 20th, 2025]
- Aveed: Enhancing Men's Health with Testosterone Replacement Therapy [Last Updated On: April 22nd, 2025] [Originally Added On: April 22nd, 2025]
- Aveed Boosts Sexual Function and Satisfaction in American Males with Hypogonadism: Study [Last Updated On: April 23rd, 2025] [Originally Added On: April 23rd, 2025]
- Aveed: Transforming Hypogonadism Treatment with Long-Acting Testosterone Therapy [Last Updated On: April 23rd, 2025] [Originally Added On: April 23rd, 2025]
- Decade-Long Study on Aveed's Cardiovascular Safety in TRT for American Males [Last Updated On: April 24th, 2025] [Originally Added On: April 24th, 2025]
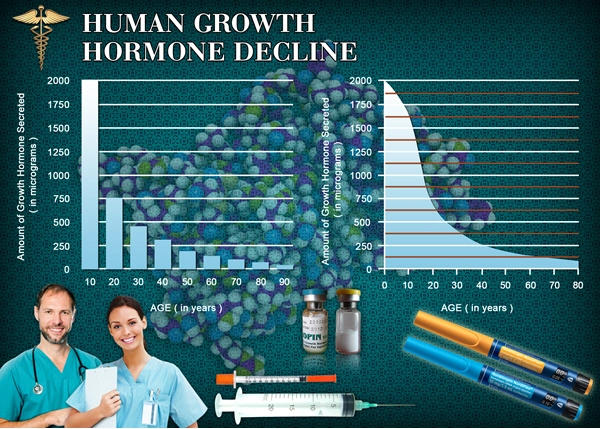
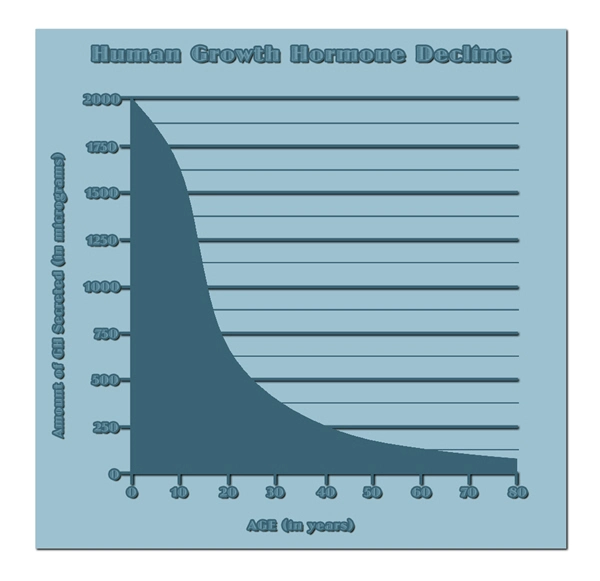
List of USA state clinics - click a flag below for blood testing clinics.
Word Count: 600


















































