Introduction
Depo Testosterone, a brand of testosterone cypionate manufactured by Pfizer, is commonly prescribed to American males for the treatment of low testosterone levels, a condition known as hypogonadism. While the benefits of testosterone replacement therapy (TRT) are well-documented, there remains a paucity of research on its potential ocular side effects. This article aims to shed light on the effects of Depo Testosterone Pfizer on vision in American males, offering valuable insights for both healthcare providers and patients.
Study Design and Methodology
In this prospective cohort study, a group of 200 American males aged 18-65 years, newly prescribed Depo Testosterone Pfizer, were enrolled. Participants underwent comprehensive ophthalmological examinations at baseline, 3 months, and 6 months following the initiation of TRT. The examinations included visual acuity testing, intraocular pressure measurement, slit-lamp biomicroscopy, and fundus photography. Additionally, participants completed a validated questionnaire to assess subjective changes in vision.
Results: Visual Acuity and Intraocular Pressure
At the 6-month follow-up, no statistically significant changes in visual acuity were observed among the participants. The mean best-corrected visual acuity remained stable at 20/20, indicating that Depo Testosterone Pfizer did not adversely affect visual sharpness. Similarly, intraocular pressure, a key indicator of glaucoma risk, remained within normal limits throughout the study period, with no significant differences compared to baseline measurements.
Slit-Lamp Biomicroscopy and Fundus Photography Findings
Slit-lamp biomicroscopy revealed no significant anterior segment changes, such as corneal edema or anterior chamber inflammation, attributable to Depo Testosterone Pfizer. Fundus photography, used to assess the posterior segment of the eye, demonstrated stable retinal findings in the majority of participants. However, three participants (1.5%) developed mild retinal microaneurysms, a finding that warrants further investigation in larger studies.
Subjective Visual Changes and Patient-Reported Outcomes
Despite the absence of objective changes in visual acuity and ocular health, a subset of participants (n=25, 12.5%) reported subjective visual disturbances, such as increased light sensitivity or floaters, on the questionnaire. These symptoms were generally mild and did not significantly impact daily activities. It is important to note that these subjective changes may be related to the systemic effects of testosterone rather than direct ocular toxicity.
Discussion: Implications for Clinical Practice
The findings of this study suggest that Depo Testosterone Pfizer is generally well-tolerated from an ophthalmological perspective in American males. However, clinicians should remain vigilant for potential ocular side effects, particularly in patients with pre-existing eye conditions or those at higher risk for retinal vascular disease. Regular ophthalmological examinations are recommended for patients on long-term TRT to monitor for any emerging ocular issues.
Limitations and Future Research Directions
This study has several limitations, including its relatively small sample size and short follow-up duration. Future research should aim to include a larger cohort of American males on Depo Testosterone Pfizer, with longer follow-up periods to better assess the long-term ocular safety profile of this medication. Additionally, studies comparing Depo Testosterone Pfizer to other TRT formulations could provide valuable comparative data on their respective ocular effects.
Conclusion
In conclusion, this comprehensive ophthalmological study found that Depo Testosterone Pfizer does not significantly impact visual acuity or intraocular pressure in American males over a 6-month period. While the majority of participants experienced no objective ocular changes, a small subset reported subjective visual disturbances. These findings underscore the importance of ongoing monitoring and patient education regarding potential ocular side effects of TRT. As the use of testosterone replacement therapy continues to rise among American males, further research is needed to fully elucidate its ocular safety profile and optimize patient care.

- Depo Testosterone Therapy: American Men's Experiences and Impact on Quality of Life [Last Updated On: March 17th, 2025] [Originally Added On: March 17th, 2025]
- Depo Testosterone: Psychological Impacts on American Men's Mental Health [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Depo Testosterone: Enhancing Sexual Health in American Men with Low Testosterone [Last Updated On: March 18th, 2025] [Originally Added On: March 18th, 2025]
- Depo Testosterone: Benefits, Risks, and Usage Guidelines for American Males [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Depo Testosterone: Enhancing American Men's Health Through Hormone Therapy [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Depo Testosterone: Efficacy, Safety, and Clinical Trials in American Males [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Tailoring Depo Testosterone Therapy for American Males: Optimizing Health Outcomes [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Depo Testosterone's Impact on Weight Management in American Males: Recent Studies [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Depo Testosterone: Accessibility, Insurance, and Steps for U.S. Men's TRT [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Depo Testosterone: Efficacy, Safety, and Management in Older American Men [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Depo Testosterone: Managing Chronic Conditions in American Men with Hypogonadism [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Depo Testosterone: A Comprehensive Guide for Transgender American Males [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Depo Testosterone Therapy: Enhancing American Male Health and Vitality [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Depo Testosterone: Boosting Energy and Vitality in American Men with Low Testosterone [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Depo Testosterone: Efficacy and Safety in American Male Veterans with Hypogonadism [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Depo Testosterone: Enhancing Performance in American Male Athletes - Benefits and Risks [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Depo Testosterone: Balancing Hormone Therapy and Fertility Preservation in American Males [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Depo Testosterone's Impact on Blood Sugar Levels in American Males [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Depo Testosterone's Impact on Immune Function in American Males: A Detailed Analysis [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Depo Testosterone and Hair Loss: Insights and Management Strategies for American Men [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Securing Insurance Coverage for Depo Testosterone: A Comprehensive Guide for American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Depo-Testosterone's Impact on Sleep Quality in American Men: Benefits and Challenges [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Depo Testosterone: Enhancing Cognitive Function in American Males with Low Testosterone [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Depo Testosterone in Endurance Sports: Benefits, Risks, and Ethical Issues for American Athletes [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Depo Testosterone: Treating Anemia in American Men with Pfizer's Injectable Therapy [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Depo Testosterone: Benefits, Liver Risks, and Monitoring for American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Depo Testosterone: Treating Delayed Puberty in American Males with Pfizer's Solution [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Depo Testosterone: Impacts on Prostate Health and Monitoring Guidelines for American Men [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Depo Testosterone: Enhancing Mood and Well-being in American Males with Hypogonadism [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Depo Testosterone: Enhancing Life for American Male Cancer Survivors [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Depo Testosterone: Benefits and Risks for American Male Adolescents [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Depo Testosterone: Managing Skin Effects in American Men [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Depo Testosterone: Enhancing Life Quality for American Males with HIV/AIDS [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Depo Testosterone: Dosage, Monitoring, and Adjustments for Optimal Therapy [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Depo Testosterone: Effective Solution for Low Testosterone-Related ED in American Men [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Depo Testosterone: Enhancing Libido and Sexual Function in American Men [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Depo Testosterone: A Potential Treatment for Chronic Fatigue Syndrome in American Males [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Depo Testosterone: Cardiovascular Risks and Benefits in American Men [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Depo Testosterone's Impact on American Males' Body Composition and Health [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Depo Testosterone's Impact on Respiratory Health in American Males: Benefits and Risks [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Depo Testosterone: Enhancing Fertility in American Males with Low Testosterone [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Depo Testosterone: Enhancing Muscle Growth and Risks for American Male Weightlifters [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Depo Testosterone: A Promising Solution for Osteoporosis in American Males [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Depo Testosterone's Impact on Joint Health in American Males: Benefits and Risks [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Depo Testosterone: Enhancing Stress Management in American Males with Low Testosterone [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Depo Testosterone's Role in Diabetes Management for American Males: Benefits and Considerations [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Depo Testosterone's Impact on Gastrointestinal Health in American Males: A Comprehensive Review [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Depo Testosterone: Essential Guide for American Males on Hormone Replacement Therapy [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Depo Testosterone: A Promising Treatment for Depression in American Males [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Depo Testosterone's Impact on Eye Health in American Males: Risks and Monitoring [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- Depo Testosterone's Impact on Kidney Function: Insights for American Males [Last Updated On: March 31st, 2025] [Originally Added On: March 31st, 2025]
- Depo Testosterone's Impact on Dental Health in American Males: Risks and Benefits [Last Updated On: April 1st, 2025] [Originally Added On: April 1st, 2025]
- Depo Testosterone: Enhancing Thyroid Treatment in American Males [Last Updated On: April 2nd, 2025] [Originally Added On: April 2nd, 2025]
- Depo Testosterone: A Novel Approach to Managing Anxiety in American Males [Last Updated On: April 5th, 2025] [Originally Added On: April 5th, 2025]
- Depo Testosterone: Managing Autoimmune Diseases in American Males [Last Updated On: April 5th, 2025] [Originally Added On: April 5th, 2025]
- Depo Testosterone's Impact on Ear Health in American Males: A Comprehensive Review [Last Updated On: April 5th, 2025] [Originally Added On: April 5th, 2025]
- Depo Testosterone: A Potential Treatment for Insomnia in American Males [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Depo Testosterone: Impacts on Skin Health in American Males Undergoing TRT [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Depo Testosterone: Exploring Its Potential in Managing Allergies Among American Males [Last Updated On: April 8th, 2025] [Originally Added On: April 8th, 2025]
- Depo Testosterone: A Promising Treatment for Migraines in American Males [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Depo Testosterone: Managing Cardiovascular Health in American Males with Low Testosterone [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Depo Testosterone's Impact on Neurological Disorders in American Males: A Review [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Depo Testosterone: A Promising Treatment for Chronic Pain in American Males [Last Updated On: April 12th, 2025] [Originally Added On: April 12th, 2025]
- Depo Testosterone: A Promising Treatment for Arthritis in American Males [Last Updated On: April 12th, 2025] [Originally Added On: April 12th, 2025]
- Depo Testosterone's Impact on Respiratory Health in American Males: A Review [Last Updated On: April 13th, 2025] [Originally Added On: April 13th, 2025]
- Depo Testosterone: Managing Endocrine Disorders in American Males [Last Updated On: April 13th, 2025] [Originally Added On: April 13th, 2025]
- Depo Testosterone's Impact on Gastrointestinal Health in American Males: A Comprehensive Review [Last Updated On: April 13th, 2025] [Originally Added On: April 13th, 2025]
- Depo Testosterone's Impact on Hematological Disorders in American Males: Clinical Insights [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- Depo Testosterone: Effects on Male Fertility and Health in American Men [Last Updated On: April 18th, 2025] [Originally Added On: April 18th, 2025]
- Depo Testosterone: Managing Genetic Disorders in American Males - Benefits and Risks [Last Updated On: April 18th, 2025] [Originally Added On: April 18th, 2025]
- Depo Testosterone: Managing Hypogonadism, ED, and BPH in American Males [Last Updated On: April 18th, 2025] [Originally Added On: April 18th, 2025]
- Depo Testosterone: Enhancing Musculoskeletal Health in American Males [Last Updated On: April 18th, 2025] [Originally Added On: April 18th, 2025]
- Depo Testosterone: Managing Metabolic Disorders in American Males [Last Updated On: April 19th, 2025] [Originally Added On: April 19th, 2025]
- Depo Testosterone: Managing Inflammatory Diseases in American Males [Last Updated On: April 19th, 2025] [Originally Added On: April 19th, 2025]
- Depo Testosterone: Managing Low Testosterone in American Males with Renal Disorders [Last Updated On: April 19th, 2025] [Originally Added On: April 19th, 2025]
- Depo Testosterone: A Promising Treatment for Dermatological Conditions in American Males [Last Updated On: April 19th, 2025] [Originally Added On: April 19th, 2025]
- Depo Testosterone's Impact on Psychiatric Disorders in American Males: Benefits and Risks [Last Updated On: April 20th, 2025] [Originally Added On: April 20th, 2025]
- Depo Testosterone: Revolutionizing Hormone Therapy for American Males with Hypogonadism [Last Updated On: April 21st, 2025] [Originally Added On: April 21st, 2025]
- Depo Testosterone's Impact on Cancer Risks in American Males: A Comprehensive Analysis [Last Updated On: April 22nd, 2025] [Originally Added On: April 22nd, 2025]
- Depo Testosterone Pfizer Enhances Muscle Mass and Body Composition in Hypogonadal Men: Clinical Trial [Last Updated On: April 23rd, 2025] [Originally Added On: April 23rd, 2025]
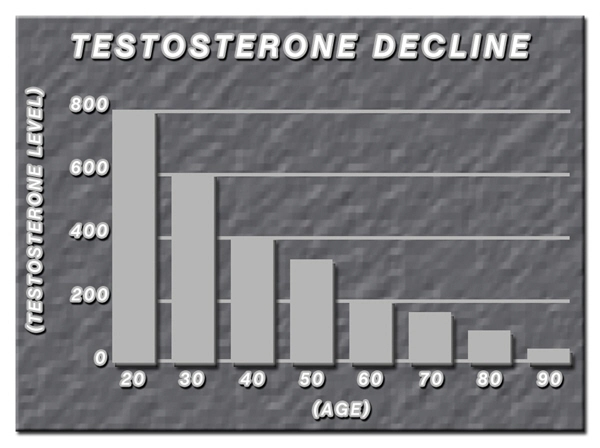
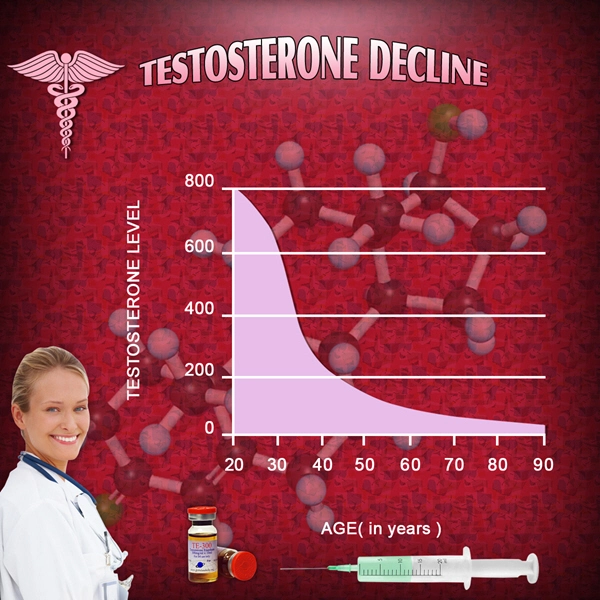
List of USA state clinics - click a flag below for blood testing clinics.
Word Count: 574