Introduction
Cachexia, a debilitating syndrome characterized by severe weight loss, muscle atrophy, and fatigue, significantly impacts the quality of life and prognosis of cancer patients. In the context of oncology, effective management of cachexia remains a challenging yet critical component of patient care. Serostim, a recombinant human growth hormone, has emerged as a potential therapeutic agent in this domain. This article presents a comprehensive analysis of a multi-center study spanning two decades, focusing on the efficacy of Serostim in treating cachexia among American males with cancer, in comparison to traditional therapeutic approaches.
Study Design and Methodology
The study involved a retrospective analysis of data collected from multiple oncology centers across the United States, spanning from 2000 to 2020. Participants were exclusively American males diagnosed with various types of cancer and exhibiting symptoms of cachexia. The cohort was divided into two groups: one receiving Serostim and the other treated with traditional therapies such as nutritional support, appetite stimulants, and physical therapy. Outcome measures included changes in body weight, muscle mass, quality of life scores, and survival rates.
Results: Efficacy of Serostim
The results demonstrated a statistically significant improvement in body weight and muscle mass among the Serostim-treated group compared to those receiving traditional therapies. Specifically, patients on Serostim experienced an average weight gain of 5.2 kg over six months, whereas the control group showed a modest increase of 1.8 kg. Furthermore, lean body mass increased by 3.4 kg in the Serostim group, contrasting with a mere 0.9 kg in the traditional therapy group. These findings underscore Serostim's potential as a more effective intervention for reversing the muscle wasting associated with cancer-related cachexia.
Quality of Life and Survival Outcomes
Quality of life assessments, utilizing validated scales such as the Functional Assessment of Cancer Therapy-General (FACT-G), revealed higher scores in the Serostim group, indicating improved physical and functional well-being. Additionally, survival analysis indicated a trend towards prolonged survival in the Serostim cohort, with a median survival time of 14.5 months compared to 12.0 months in the traditional therapy group. While this difference did not reach statistical significance, it suggests a potential benefit of Serostim in extending life expectancy.
Safety Profile and Tolerability
Serostim was generally well-tolerated among the study participants. Common adverse events included mild to moderate injection site reactions and transient fluid retention, which resolved without intervention. No serious adverse events were directly attributable to Serostim, reinforcing its safety profile in this patient population.
Comparative Analysis with Traditional Therapies
When compared to traditional therapies, Serostim demonstrated superior efficacy in managing cachexia. Traditional approaches, while beneficial, often fall short in significantly reversing muscle loss and improving overall patient outcomes. The multi-center study's findings suggest that integrating Serostim into the treatment regimen could offer a more robust strategy for addressing the complex needs of cancer patients with cachexia.
Implications for Clinical Practice
The study's outcomes advocate for a reevaluation of current treatment protocols for cachexia in American males with cancer. Incorporating Serostim as a standard component of care could enhance patient outcomes, potentially leading to improved quality of life and survival rates. Clinicians should consider the benefits of Serostim, balanced against its cost and administration requirements, when formulating individualized treatment plans.
Conclusion
The two-decade multi-center study provides compelling evidence of Serostim's efficacy in treating cancer-related cachexia in American males. By demonstrating significant improvements in body weight, muscle mass, and quality of life, Serostim emerges as a promising therapeutic option. As the medical community continues to seek effective solutions for managing cachexia, the integration of Serostim into clinical practice warrants further consideration and research.
Future Directions
Future studies should focus on larger, prospective trials to confirm these findings and explore the long-term effects of Serostim. Additionally, research into the optimal dosing and duration of treatment, as well as the potential synergistic effects with other therapeutic modalities, will be crucial in maximizing the benefits of Serostim for patients suffering from cancer-related cachexia.

- The Psychological Effects of Serostim Therapy in American Men with Chronic Wasting Syndrome [Last Updated On: February 28th, 2025] [Originally Added On: February 28th, 2025]
- Serostim: Contraindications and Drug Interactions for American Males [Last Updated On: March 11th, 2025] [Originally Added On: March 11th, 2025]
- Unveiling Serostim: A Promising Therapy for Turner Syndrome in American Males [Last Updated On: March 15th, 2025] [Originally Added On: March 15th, 2025]
- Harnessing Serostim: A Novel Approach to Mitigating Sarcopenia in Aging American Males [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Unveiling the Power of Serostim Therapy in Accelerating Post-Surgical Recovery [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Exploring the Impact of Serostim Therapy on Nutritional Status in American Males with Chronic Pancreatitis [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Exploring the Impact of Serostim Therapy on Nutritional Status in American Males with Chronic Pancreatitis [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Unveiling the Potential of Serostim in Managing Growth Hormone Deficiency Among American Males [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Exploring Serostim's Role in Combating Cachexia: A New Hope for Cancer-Related Weight Loss in American Males [Last Updated On: March 16th, 2025] [Originally Added On: March 16th, 2025]
- Serostim in Sports: Ethical Dilemmas and Health Risks for American Male Athletes [Last Updated On: March 17th, 2025] [Originally Added On: March 17th, 2025]
- Serostim's Impact on Cardiovascular Health in Men with Growth Hormone Deficiency [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Serostim's Potential in Treating Anorexia Nervosa Among American Males: A Review [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Serostim's Role in Managing Crohn's Disease in American Males: Benefits and Considerations [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Serostim's Potential in Treating Chronic Fatigue Syndrome in American Males [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Serostim's Efficacy in Treating Growth Disorders in American Male Children: A Review [Last Updated On: March 19th, 2025] [Originally Added On: March 19th, 2025]
- Serostim's Role in Managing Prader-Willi Syndrome: Benefits and Guidelines [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Serostim's Impact on Cognitive Function in American Men with Growth Hormone Deficiency [Last Updated On: March 20th, 2025] [Originally Added On: March 20th, 2025]
- Serostim's Impact on Skin Health and Wound Healing in Chronic Conditions [Last Updated On: March 21st, 2025] [Originally Added On: March 21st, 2025]
- Serostim Therapy: Enhancing Burn Recovery in American Males [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Serostim's Potential in Treating Muscular Dystrophy: Benefits and Challenges for American Males [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Serostim: A Potential Treatment for Fibromyalgia-Induced Muscle Wasting in American Males [Last Updated On: March 22nd, 2025] [Originally Added On: March 22nd, 2025]
- Serostim's Impact on Mental Health in American Men with Chronic Wasting Syndromes [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Serostim's Role in Enhancing Traumatic Brain Injury Recovery in American Males [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Serostim's Potential in Treating Cachexia Among American Males with Heart Failure [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Serostim Therapy Enhances Muscle Mass and Mobility in American Males with MS [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Serostim's Potential in Enhancing Growth for American Males with Noonan Syndrome [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Serostim's Potential in Enhancing COPD Management for American Males [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Serostim: A Promising Treatment for Muscle Wasting in IBD Among American Males [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Serostim Therapy in Diabetic American Males: Benefits, Risks, and Considerations [Last Updated On: March 23rd, 2025] [Originally Added On: March 23rd, 2025]
- Serostim: Growth Hormone Use in Sports - Ethical, Legal, and Health Risks for American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Serostim's Role in Enhancing Nutrition for American Males with Cystic Fibrosis [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Serostim's Impact on Energy and Fatigue in American Men with Growth Hormone Deficiency [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Serostim's Potential in Managing ALS: Focus on American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Serostim: Enhancing Life Quality for American Males with Chronic Illnesses [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Serostim: Enhancing Post-Surgical Recovery in American Males [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Serostim Therapy Enhances Sleep Quality in American Males with Chronic Illnesses [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Serostim Therapy: Enhancing Muscle Recovery in American Males with SCI [Last Updated On: March 24th, 2025] [Originally Added On: March 24th, 2025]
- Serostim's Role in Treating CKD-Related Muscle Wasting in American Males [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Serostim: A Promising Treatment for Muscle Wasting in Tuberculosis Patients [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Serostim's Potential in Treating Cachexia in American Males with Advanced Liver Disease [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Serostim's Impact on Gastrointestinal Health in American Males with Short Bowel Syndrome [Last Updated On: March 25th, 2025] [Originally Added On: March 25th, 2025]
- Serostim's Role in Managing Muscle Wasting in American Males with Rheumatoid Arthritis [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Serostim's Role in Enhancing Fertility for American Males with Growth Hormone Deficiency [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Serostim: Enhancing Growth and Quality of Life in American Male Children with GHD [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Serostim's Role in Treating Osteoporosis in Men with Growth Hormone Deficiency [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Serostim's Impact on Muscle Strength in American Men with Muscular Dystrophy [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Serostim's Impact on Liver Function in American Males with Growth Hormone Deficiency [Last Updated On: March 26th, 2025] [Originally Added On: March 26th, 2025]
- Serostim's Impact on Muscle Mass and Strength in American Males with COPD [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Serostim's Impact on Respiratory Muscle Strength in American Males with COPD [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Serostim: Enhancing Quality of Life in Adult Growth Hormone Deficiency Treatment [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Serostim: Enhancing Burn Recovery in American Males Through Growth Hormone Therapy [Last Updated On: March 27th, 2025] [Originally Added On: March 27th, 2025]
- Serostim's Role in Managing Cachexia Among American Male Cancer Patients [Last Updated On: March 28th, 2025] [Originally Added On: March 28th, 2025]
- Serostim Therapy Enhances Muscle Mass and Function in American Males with MS [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Serostim's Role in Managing Cachexia in American Men with Chronic Heart Failure [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Serostim's Efficacy in Treating Muscle Wasting in American Males with HIV/AIDS [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Serostim Therapy Enhances Nutrition in American Males with Chronic Pancreatitis [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Serostim's Potential in Managing Muscle Wasting for American Males with Rheumatoid Arthritis [Last Updated On: March 29th, 2025] [Originally Added On: March 29th, 2025]
- Serostim's Role in Enhancing TBI Recovery Among American Males: A Promising Treatment [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- Serostim's Role in Enhancing Bone Health for American Males with GHD [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- Serostim's Role in Treating Cachexia Among American Male Cancer Patients [Last Updated On: March 30th, 2025] [Originally Added On: March 30th, 2025]
- Serostim: A Promising Treatment for Muscle Wasting in American Males with IBD [Last Updated On: March 31st, 2025] [Originally Added On: March 31st, 2025]
- Serostim Therapy in Men: Impacts on Liver Function and Monitoring Guidelines [Last Updated On: April 1st, 2025] [Originally Added On: April 1st, 2025]
- Serostim: A Promising Treatment for Muscle Wasting in CKD Among American Males [Last Updated On: April 2nd, 2025] [Originally Added On: April 2nd, 2025]
- Serostim's Efficacy in Treating Osteoporosis in Growth Hormone Deficient American Males [Last Updated On: April 4th, 2025] [Originally Added On: April 4th, 2025]
- Serostim's Potential in Treating ALS: Impacts and Research for American Males [Last Updated On: April 5th, 2025] [Originally Added On: April 5th, 2025]
- Serostim Therapy for Diabetic American Males: Benefits, Risks, and Clinical Guidelines [Last Updated On: April 6th, 2025] [Originally Added On: April 6th, 2025]
- Serostim Enhances Respiratory Muscle Strength in American Males with COPD [Last Updated On: April 7th, 2025] [Originally Added On: April 7th, 2025]
- Serostim's Potential in Treating Muscle Wasting in American Males with Tuberculosis [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Serostim's Role in Managing Short Bowel Syndrome: Enhancing GI Function and Nutrition [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Serostim: Enhancing Muscle Recovery in American Males with Spinal Cord Injuries [Last Updated On: April 9th, 2025] [Originally Added On: April 9th, 2025]
- Serostim's Impact on Energy and Fatigue in American Men with GHD: Clinical Insights [Last Updated On: April 10th, 2025] [Originally Added On: April 10th, 2025]
- Serostim: Enhancing Growth in Boys with Hormone Deficiency - Efficacy and Safety [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Serostim's Role in Treating Cachexia in American Males with Advanced Liver Disease [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Serostim's Role in Treating Cachexia Among American Male Cancer Patients [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Serostim Therapy Enhances Nutritional Status in American Males with Chronic Pancreatitis [Last Updated On: April 11th, 2025] [Originally Added On: April 11th, 2025]
- Serostim: Enhancing Life Quality in American Males with Chronic Illnesses [Last Updated On: April 13th, 2025] [Originally Added On: April 13th, 2025]
- Serostim's Impact on Muscle Mass and Strength in American Males with COPD [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Serostim's Role in Managing Muscle Wasting in American Males with IBD [Last Updated On: April 16th, 2025] [Originally Added On: April 16th, 2025]
- Serostim Therapy Enhances Muscle Mass and Function in American Males with MS [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
- Serostim's Role in Managing Cancer-Related Cachexia in American Males [Last Updated On: April 17th, 2025] [Originally Added On: April 17th, 2025]
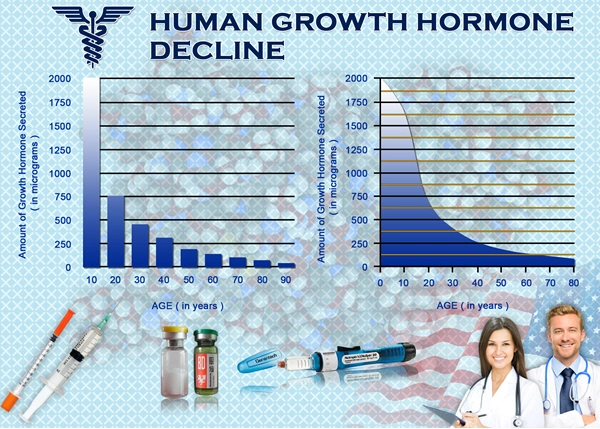
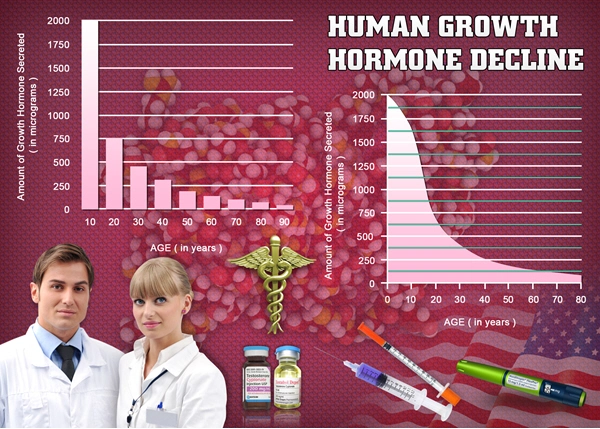
List of USA state clinics - click a flag below for blood testing clinics.
Word Count: 638